Fredrik Almqvist
The research interests in Fredrik Almqvist’s (FA) lab focus on method developments in organic synthesis and design and synthesis of molecules that interact with macromolecules, in particular inhibition of protein-protein interactions. Examples of the latter involve development of inhibitors of bacterial macromolecular assembly and amyloid formation. The FA lab has pioneered the design and synthesis of ring-fused 2-pyridone compounds as peptidomimetics and together with Dr. Hultgren’s lab at Washington University in St. Louis they have shown that these mimetics are ideal for the development of antivirulence compounds. FA has developed small molecules called “pilicides” that inhibit chaperone-usher pathway (CUP) pili, which are important virulence factors of uropathogenic E. coli (UPEC) and this has resulted in a deeper understanding of regulation of CUP pili biosynthesis and the ability of UPEC to establish biofilm formation. In addition, together with Dr. Chapman at the University of Michigan FA have further studied a subset of the pilicides called “curlicides” that dependent upon the substitution pattern on the central fragment have the ability to affect curli assembly in E. coli. These compounds are excellent tools in the strive for a greater understanding of the mechanisms behind amyloid assembly in general but curli assembly in particular. Both pili and curli are important constituents in different bacterial biofilms and the group has a general interest in developing biofilm inhibitors. In this context FA started a few years ago a collaboration with Dr. Christina Stallings at Washington University in St. Louis to develop their discovery of mycobaterial tolerance inhibitors (MTIs) and investigate and understand the exact mode of action of the MTIs. This discovery was the starting point of the JPIAMR funded project “MTI4MDR” where research groups in Europe led by Dr. Priscille Brodin in Lille, Dr. Jesus Blazques in Madrid and Dr. Tone Tønjum in Oslo have joined forces to study the scope and limitations of this new class of antitubercular compounds. These new compounds have great potential to improve treatment regimens for Tuberculosis, the deadliest infectious disese in the world. The wide range of inhibitors that have been developed in the lab has led to several cross disciplinary collaborations and among these FA has ongoing research with UCMR researchers Dr. Sven Bergström and Åsa Gylfe (Chlamydia), Jörgen Johansson and Elisabeth Sauer-Eriksson (Listeria) and Johan Normark (Tuberculosis) with the aim to improve efficacy and disect exact mode of action.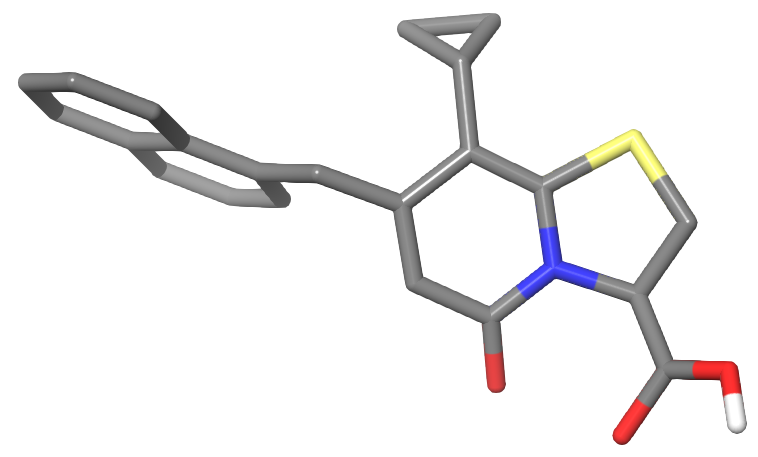

Priscille Brodin
“Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.”

Tone Tønjum
“Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.”

Jesús Blázquez
The research of the Jesús Blázquez´s (JB) group of “Stress and Bacterial Evolution”, at the National Centre for Biotechnology (CNB-CSIC), focus on the genetic mechanisms involved in bacterial genome stability and their roles in evolution and adaptation. The JB lab interest is to understand the genetic basis of both stable and induced hyper-mutation/hyper-recombination as bacterial “strategies” to speed adaptation to stress, particularly to antibiotics. Recently, the JB group described the genetic characteristics of a novel non-canonical mismatch repair (nc-MMR) system in some Prokaryotes, including Mycobacteria, key for maintaining their genome stability. Currently, JB group is trying to disentangle the possible relationship between genome stability/instability andantibiotic resistance development in pathogenic Mycobacteria, including M. tuberculosis, M. abscessus and M. avium. From a practical perspective, the JB lab wants to apply this knowledge to i) prevent and fight antibiotic resistance in bacterial pathogens by searching for new antibiotics (such as anti-mutagenic molecules and inhibitors of β-lactamases), and ii) engineer prokaryotic species (eg Streptomyces) to improve their biotechnological interest. Under the umbrella of the JPIAMR funded project “MTI4MDR” and in collaboration with F. Almqvist (Umeå University), P. Brodin (Institut Pasteur Lille), T. Tonjum (University of Oslo and Rikshospitalet) and C. Stallings (Washington University), the JB lab is analyzing the activity and mode of action of mycobaterial tolerance inhibitors (MTIs) molecules, developed by Fredrik Almqvist´s lab, against M. abscessus and M. avium.

Christina Stallings
“Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.”